Ushering the era of Decentralised Oncology Clinical Trials in India
By Bharat Kumar Sarvepalli, Assistant Vice President, Karkinos Healthcare
India has a huge potential for conducting global clinical trials and accelerated drug discovery. The Indian clinical trials market size, according to Grand View Research, was valued at USD 1.93 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 8.2% from 2022 to 2030. This growth can be attributed to many factors like the increasing prevalence of cancer in the country; supporting clinical trial infrastructure; regulatory reforms moving towards more open access to clinical trials; and the growing demand for advanced and personalized medicine.
The other major attributable reason to a flourishing clinical trials market in India is the digitalisation of clinical trials, which has enabled streamlining of several trial processes such as patient data capture, remote patient monitoring, near home access, and streamlined logistics and supply-chain management.
What really sets India on the advantage path is the fact that clinical trials can be done in a more cost-effective manner in this country. Another major driver that is accelerating the pace of new clinical trials in India is the optimum use of latest technologies and its deployment in the trial sites. From telehealth to Electronic Health Records (EHRs), technology-based tools are empowering the patient and the physician to take better charge of health conditions in a more sophisticated manner and most essentially cutting down on time lags for quicker response to save lives.
What are Decentralised Clinical Trials?
Decentralised clinical trials (DCTs) are a modern approach to conducting clinical research that leverages digital technologies and remote monitoring methods. In traditional clinical trials, patients are typically required to visit specific study sites, such as hospitals or research centres, to receive treatments, undergo tests, and have their health monitored by healthcare professionals.
A decentralised approach, therefore, enables clinical trial activities to be conducted in the comfort of patients’ homes, reducing the need for frequent visits to a clinical site. This not only improves participant recruitment and retention but also enhances the diversity and inclusivity of the study population. Additionally, decentralised clinical trials (DCTs) have the potential to generate real-world evidence that better reflects patient’s everyday experiences and healthcare outcomes.
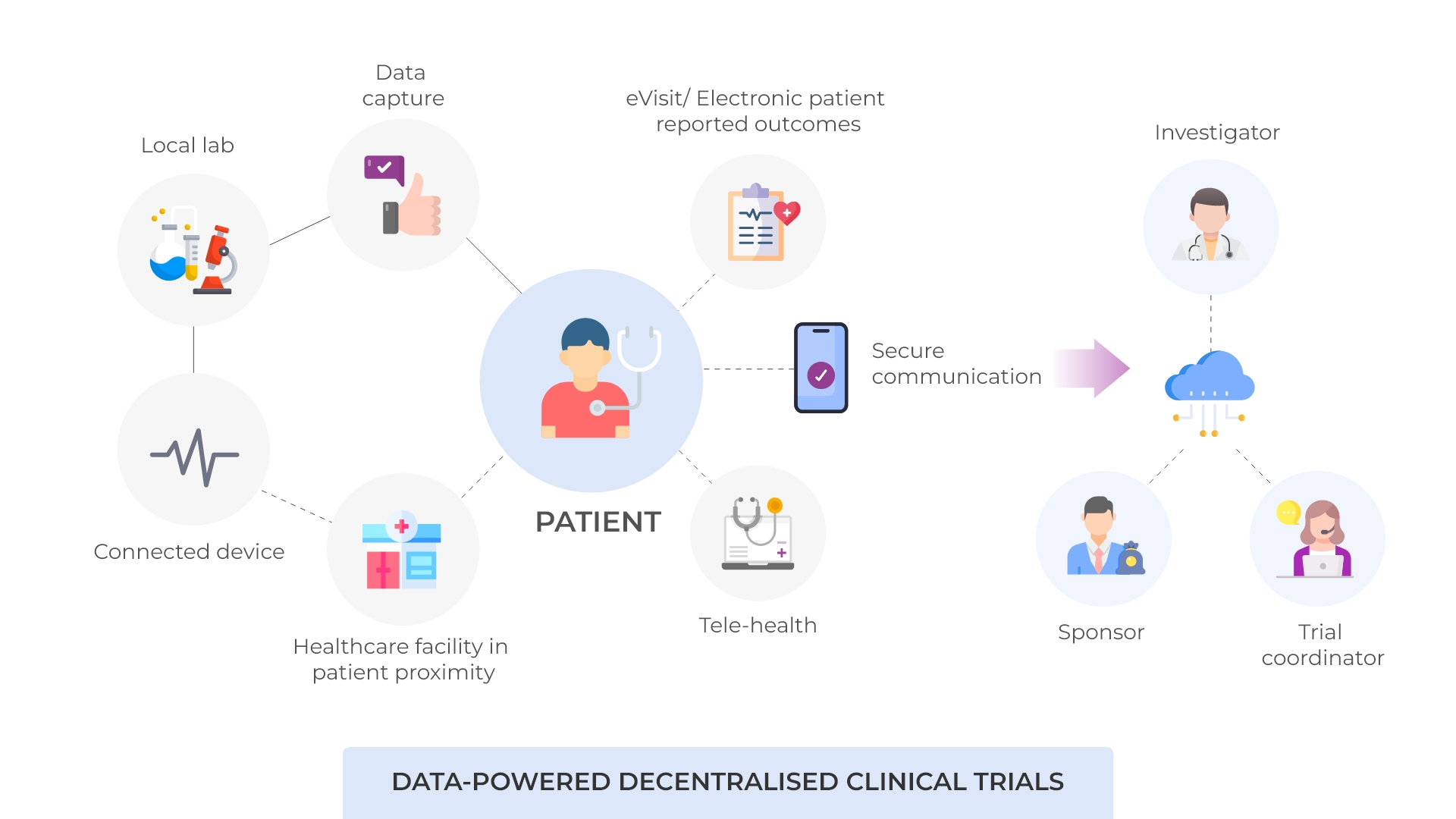
Karkinos Healthcare is a pioneer in harnessing technology to enable DCTs in India. Through its distributed cancer care network (DCCN) and a robust digital oncology platform, Karkinos is facilitating DCT services, at a pan-India level, in the most comprehensive and cost-effective manner.
This article brings to fore the expertise of Karkinos Healthcare in providing DCT services in bringing about novel approaches to cancer treatment. To know more about the compelling reasons for the emergence of Decentralised Clinical Trials (DCTs) in India and what makes the country a hotbed for global pharmaceutical companies to conduct DCTs, please subscribe to the full article by accessing it at https://karkinos.in/clinicaltrials/.
